A few weeks ago, T2 (you know who you are) asked me the above question. At the time, I gave a rather brusque reply that esterification is not a redox reaction as the oxidation state did not change. (That was because I was feeling devastated after a (non-chemistry) lesson.. Don’t worry, if you’re reading this post, I am most probably not referring to you.. But I digress.) Anyways, the question deserves to be answered in greater detail! So here it is!
The diagram below shows an esterification reaction between methanol and ethanoic acid to form methyl ethanoate. We will use this example to illustrate why esterification is not a redox reaction.
Eagle-eyed observers would note that methanol has lost a hydrogen (i.e. oxidized), this should imply that ethanoic acid is reduced and hence the reaction is a redox reaction. However, the best method of determining if a reaction is redox is to check the oxidation states/determine if electrons are gained or lost. The other two definitions are approximations used at O levels to make your life simplier. They fail at explaining complex scenarios such as this. In another (yet to be written) post, I will explain why it is generally ok to use the other two definitions in most situations.
Rules for assignment of oxidation states
As you know, in an ionic compound, the charge of the ion is the oxidation state. For example, the o.s. of Na in NaCl is +1.
However, for covalent compounds, the more electronegative atom is assigned a negative oxidation state (because it has a higher affinity for electrons) while the less electronegative atom is assigned a positive oxidation state. If they form a single covalent bond, the more electronegative atom is assigned an o.s. of -1 while the other atom is assigned an o.s. of +1.
Take for example, a compound formed between fluorine and chlorine (FCl). Fluorine being more electronegative will be assigned an oxidation state of -1 while chlorine being less electronegative is assigned an oxidation state of +1.
So how does this apply to esterification?
To begin with, please note that the oxidation state of the oxygen atom on methanol is “-1” as it is more electronegative (ENC of oxygen = 6, ENC of hydrogen = 1) than the hydrogen atom that it is bonded to. See diagram below.
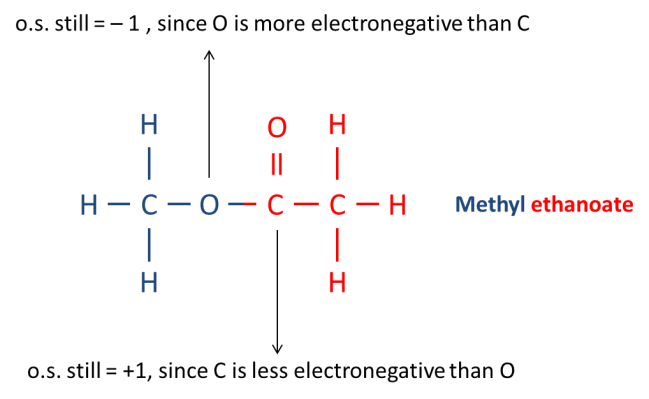
On the other hand, for ethanoic acid, the oxidation state of the carbon atom on the carbonyl group is “+1” as it is bonded to a more electronegative oxygen atom (ENC of oxygen =6, ENC of carbon =4). See diagram below.
After esterification, the oxygen from the alcohol forms a single covalent bond with the carbonyl functional group of the ethanoic acid as shown below.
Note that oxygen is more electronegative than carbon. Hence, the o.s. of oxygen remains as “-1” while the o.s. of carbon remains as “+1”. Since there is no change in o.s., esterification is not considered a redox reaction!