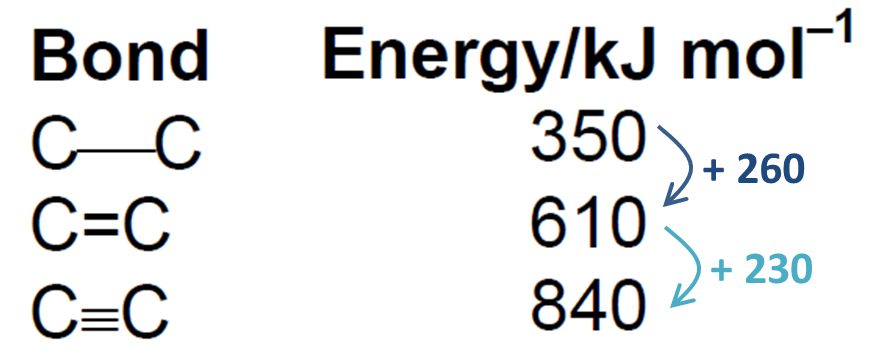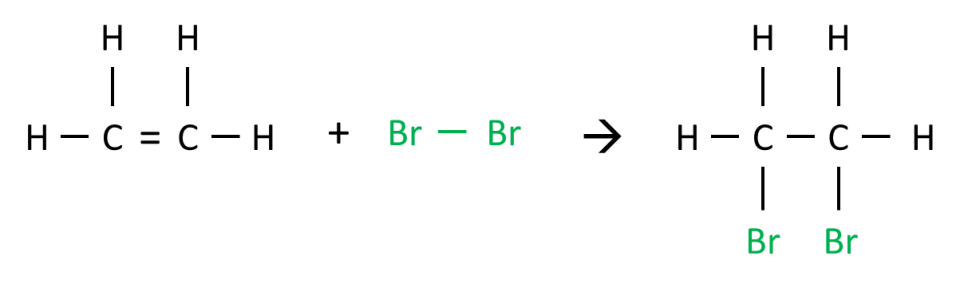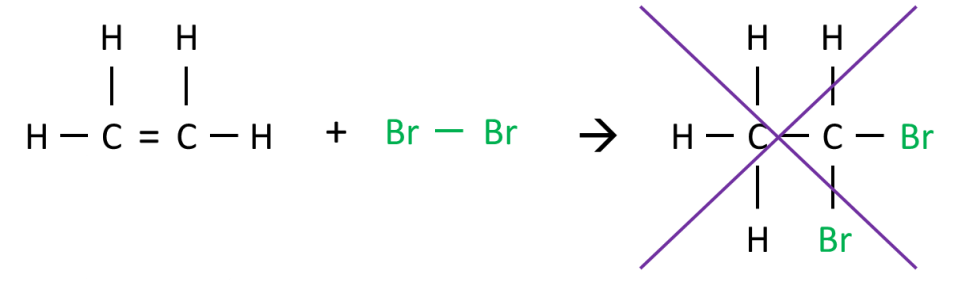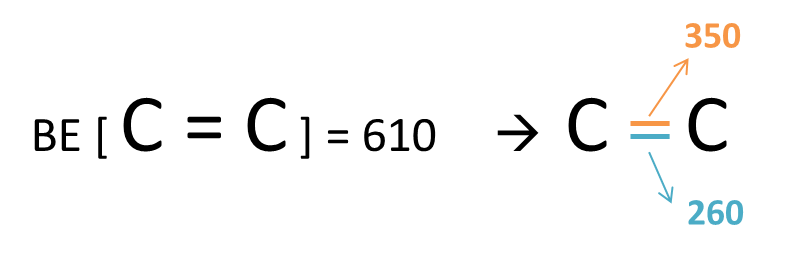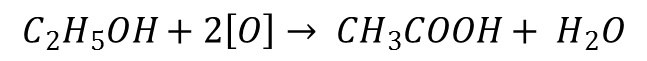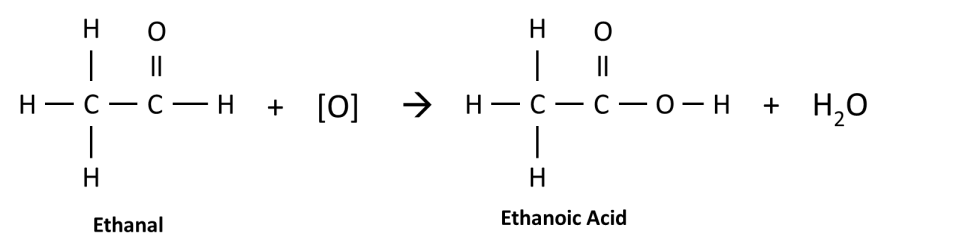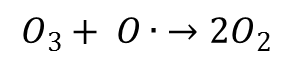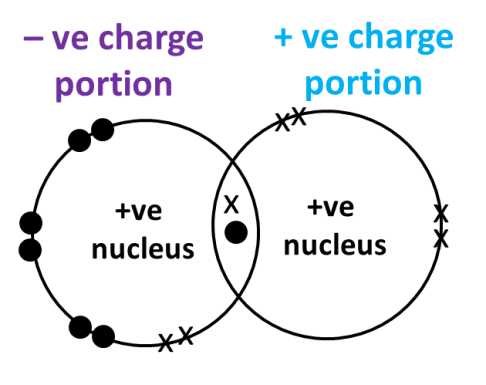Home » Uncategorized (Page 2)
Category Archives: Uncategorized
Electronegativity!
Electronegativity refers to the ability of an atom to attract electrons toward itself. A more electronegative atom has higher ability to attract electrons towards itself.
Electronegativity depends on two factors
- Effective Nuclear Charge (ENC)
- Distance from nucleus
To summarize:
- ↑ Effective Nuclear Charge (ENC) = ↑ Electronegativity
- ↑ Distance from nucleus = ↓ Electronegativity
Effective Nuclear Charge ≈ No. of protons – No. of inner shell shielding electrons
This is an intuitive formula. The protons in the atom are trying to attract electrons (unlike charges attract) while the inner shell electrons are trying to repel away electrons that are being added to the valence shell (like charges repel). Thus, ENC measures the net attraction that an atom has on the valence electrons.
For example, Fluorine (electronic arrangement: 2,7) is more electronegative than Oxygen (2,6).
ENC of F = 9 – 2 = 7
ENC of O = 8 – 2 =6
Therefore, it can be concluded from the above example that electronegativity increases across a period, from Group I to Group VII. Group 0 is excluded as it has stable electronic arrangement, hence will not form bonds.
How about elements from the same group? Don’t they have the same ENC?
Yes, you are right! Elements of the same group have the same ENC. For instance Fluorine (2,7) and Chlorine (2,8,7)
ENC of F = 9 – 2 = 7
ENC of Cl = 17 – 10 = 7
To compare elements in the same group, we have to use distance from the nucleus. Chlorine has 3 electron shells as compared to 2 for Fluorine. Thus, the valence shell of Chlorine is further away from the positive nucleus as compared to Fluorine.
The greater distance between the positive nucleus and the valence shell makes it more difficult for Chlorine to attract electrons as compared to Fluorine. Hence, Chlorine is less electronegative as compared to Fluorine.
Therefore, it can be concluded from the above example that electronegativity decreases down the group!
Putting these two factors together, you should be able to conclude for yourself that Fluorine is the most electronegative atom while Francium is the least electronegative! (For A level students, I am basing this on the Pauling scale.)
The curious case of the carbon – carbon bond strengths
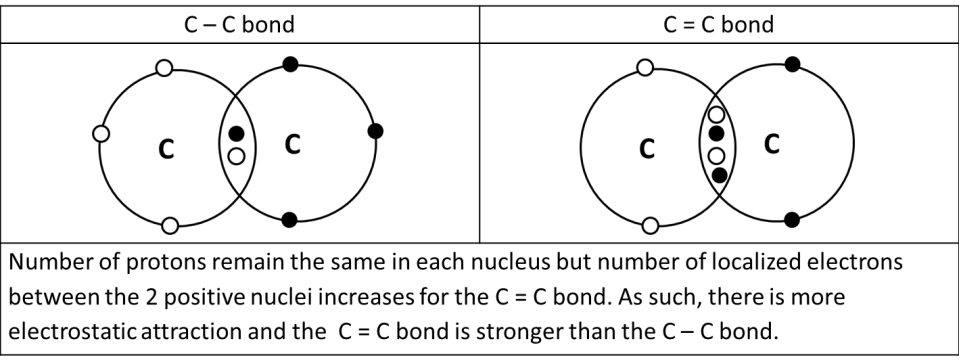
Readers of previous posts will (hopefully) remember that the covalent bond is defined as the electrostatic force of attraction between the localized shared pair of electrons and the 2 positively charged nucleus.
Since electrostatic forces of attraction depend on the difference in charges, a double bond is going to be stronger than a single bond and a triple bond is going to be stronger than a double bond. For instance, MgO has a higher m.p. as compared to NaF because there is a greater difference in the charges between the Mg2+ and O2– ions as compared the Na+ and F– ions, hence the electrostatic forces of attraction between the Mg2+ and O2– ions are stronger and require more energy to break, resulting in higher m.p.
The same holds true for covalent bonds. Thus, the C ≡ C bond is stronger than the C = C bond. In turn, the C = C bond is stronger than the C – C bond. See diagram below.
However, closer inspection of the bond energies indicates that the increase in bond strength is non-linear in nature. In fact, it is increasing at a decreasing rate!
Tell me why!
As the number of electrons in the same space increases, there would be more repulsion between the electrons (since like charges repel), resulting in a lower than proportionate increase in the bond strength as the number of bonds increases.
Note: JC students should know that the “proper explanation” should include a discussion about the sigma, pi bonds and their different degrees of overlap of the electron cloud. However, the basic idea remains the same. There is less overlap for pi bonds because electrons are arranged in such a way so as to minimize repulsion. Even then, the 2 pi bonds are not of equal strength, suggesting that there remains some degree of electron repulsion.
Why can’t both bromine atoms be added to the same carbon atom during addition reaction with alkenes?
As you guys know, the product of bromination should be drawn like this:
Instead of something like:
The reason for this lies in the way the addition reaction takes place
To be precise, this mechanism is known as “electrophilic addition” and applies to addition of electrophiles such as halogens (e.g. bromine) and water to alkenes. This mechanism does not apply to catalytic hydrogenation which will be covered in another post.
What are “electrophiles”?
Electrophiles simply refer to molecules/ions that “like electrons”. On the other hand, an electrophobe is a molecule/ion that “dislikes electrons”.
Mechanism
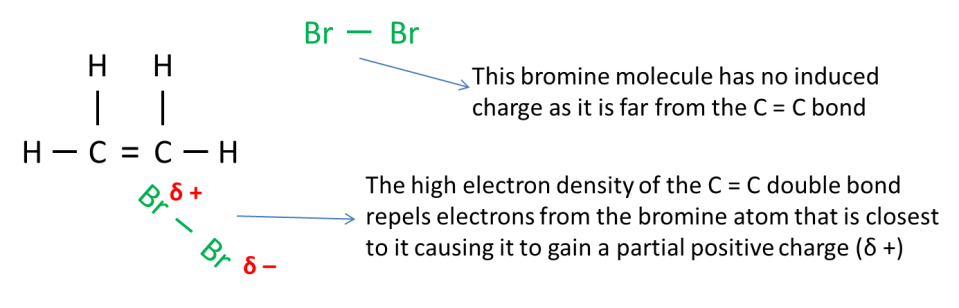
The C = C portion of the alkene is said to have “high electron density” / “electron-rich” as there are 4 electrons that are clustered around this region. Therefore when a bromine molecule is brought near to the C = C portion of the alkene, the electrons on the bromine atom that is closer to C =C bond will be repelled to the other bromine atom because like charges repel.
The bromine atom that is nearer to the C = C bond acquires a partial positive charge (δ +) while the other bromine atom becomes partially negative (δ – ). This is illustrated in the diagram below.
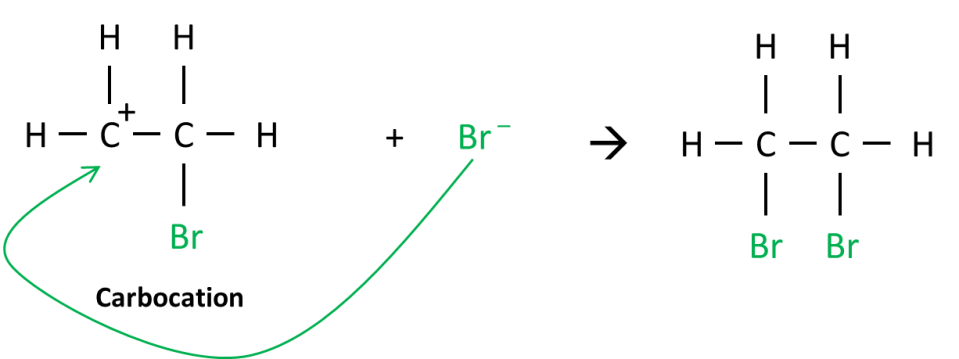
The partially positive bromine atom is attracted to the “electron-rich” C = C double bond. A pair of electrons is transferred from the C = C bond to the partially positive bromine atom forming a C – Br bond with one of the carbon atoms. The C = C bond becomes a C – C bond. This results in the formation of an intermediate known as a carbocation (think carbon – cation) and a negative bromine ion. See diagram below.
Finally, the negative bromine ion is attracted to the carbocation forming the final product, 1,2-dibromoethane!
Tell me why! (A pair of electrons are transferred from the C = C bond to the partially positive bromine atom..)
As you guys know, most chemical reactions are driven by the quest for greater stability. The C = C bond [BE= 610kJ/mol] while stronger than the C – C bond [BE = 350kJ/mol] is less than twice as strong. You can artificially decompose the C = C bond into two C – C bonds as shown below.
Why is one bond weaker than the other? Since like charges repel each other, you can think of the reason for this as due to higher levels of repulsion as there are more electrons that are “clustered” in the same space. [JC students should think in terms of sigma and pi bonds. Just saying.]
Hence when the partially positive bromine atom that is deficient in electron approaches the C = C bond, the pair of electrons from the C = C bond which is less stable [BE = 260kJ/mol] will “defect” to it, forming the C – Br bond [BE = 280kJ/mol], which is stronger, hence achieving a higher degree of stability!
Why must the oxidizing agent be in excess during the oxidation of alcohol to carboxylic acid?
Okay.. Minor things first.. As you guys know, for simplicity’s sake, the oxidation of alcohols to carboxylic acids using an oxidizing agent such as acidified potassium dichromate (K2Cr2O7) is represented by an equation that looks like this:
However, I detected dissent from people in class.. Like.. Why don’t we write the full equation? What so difficult about it? Tell me why?! That is because the full equation looks something like:
I’m sure u guys agree that it is better that we stick to the simplified form.. no?
Anyways.. Moving on.. Why must the oxidizing agent be in excess?
That is because the oxidation of alcohols to carboxylic acids takes place via a 2 stage process.
In stage 1:
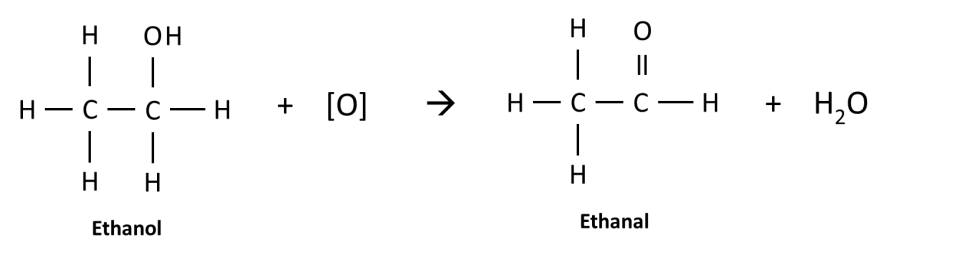
Alcohols are oxidized to form aldehydes. In the example below, ethanol is oxidized to an aldehyde called ethanal.
In stage 2
The aldehyde is then oxidized to form the carboxylic acid. In the example below, ethanal is oxidized to form ethanoic acid.
If there is insufficient oxidizing agent, the reaction stops at the aldehyde instead of forming the desired carboxylic acid! Similarly, reflux condition is needed to prevent the ethanal from escaping before it is completely oxidized to form the carboxylic acid!
Note: For JC students, this mechanism applies to primary alcohols. Often, secondary alcohols do not fully oxidize to form carboxylic acids but stop at ketones.
Why is aluminium chloride covalent?
To keep a long story short, aluminium chloride exhibits covalent character because
- The aluminium ion has high charge density
- The electron cloud of the chloride is distortable (JC term, polarizable)
- The combination of the 2 factors above leads to some degree of electron sharing between the positive aluminium ion and the negative chloride ion and hence the covalent character.
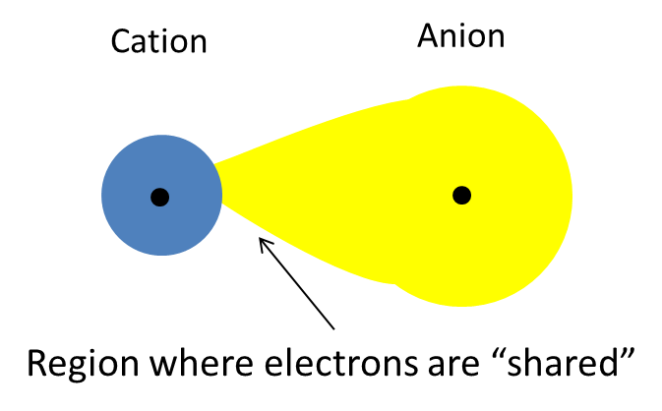
In general, in order for an ionic compound to have covalent character (oxymoronic, I know.. but.. ) the positive charge of the cation must be so strong that it causes the electron cloud of the anion to be distorted (fancy JC term, polarized) such that electrons become drawn into the space between the positive cation and the negative anion. Hence, the electrons are said to be “shared” and the ionic compound is said to exhibit some covalent character. (See diagrams below)
Scenario 1: Large cation = lower charge density, not enough to distort the electron cloud of the anion significantly.
 Scenario 2: As the charge density of the cation increases (represented by smaller cation), the attractive power of the cation increases and the electron cloud of the anion becomes increasingly distorted.
Scenario 2: As the charge density of the cation increases (represented by smaller cation), the attractive power of the cation increases and the electron cloud of the anion becomes increasingly distorted.
Scenario 3: When the charge density of the cation is high (represented by a small cation), the attractive power of the cation is strong enough to distort the electron cloud of the anion such that some electrons are “shared” between the 2 ions.
Let’s take a deeper look at the 2 factors described briefly above:
- “Strength” of cation (JC term, polarizing power of cation)
- Ease of distortion of electron cloud of anion (JC term, polarizability of anion)
“Strength” of cation
“Strength” of cation is related to its charge density, which can be loosely defined as charge divided by volume of cation. The larger the charge density, the more “concentrated” the positive charge (the cation becomes more “powerful” so to speak), the more likely it is able to attract electrons from the anion into the space between the cation and the anion, thereby creating “electron sharing” and hence covalent character.
Why does aluminium ion have high charge density?
Firstly, aluminium ions have high charge of +3. Also, as compared to sodium or magnesium, the ionic size of aluminium is smaller (All three ions have electronic structure 2,8. However, Al has 13 protons in the nucleus vs 12 and 11 for Mg and Na respectively. Hence the electrons of Al would be pulled closer to the nucleus due to the greater number of protons in the nucleus resulting in a smaller ionic size). Since, aluminium ion has high charge, low volume it has high charge density.
Ease of distortion of electron cloud of anion
Ease of distortion of electron cloud of anion depends on
- Size of anion
- Charge of anion
Factor 1 is intuitive. The larger the size of the anion, the further away the valence shell electrons will be from the positive nucleus, the lesser the amount of electrostatic attraction between the positive nucleus and the valence shell electrons, the easier it will be for the valence electrons to be pulled away by the positive cation causing the electron cloud to be distorted.
Comparing the electronic structures of Fluoride (2,8) and chloride (2,8,8) you will realize that chloride has one extra electron shell, hence, the valence electrons of chloride will be further away from the positive nucleus as compared to fluoride and it becomes easier for the valence electrons to be attracted away by a positive cation.
For factor 2, a higher negative charge on the anion implies that it is easier for the electron cloud to be distorted. We explain this using the example of chloride and sulphide ions (electronic structure of both: 2,8,8). Chlorine has 17 protons while sulphur only has 16. Thus as compared to chlorine, sulphur will exert less electrostatic attraction on its valence electrons, making it easier for them to be pulled away by a positive cation.
Putting the factors together:
Aluminium chloride is covalent because the aluminium ion has high charge density while the chloride ion is relatively polarizable. Extending this concept, you should find that aluminium bromide, aluminium iodide would all exhibit covalent character. Furthermore, because the number of electron shells is increasing, the degree of covalent character would increase as well (because it is even easier for the electron cloud to be distorted. See factor 1. This leads to a greater degree of “electron sharing” and hence a greater degree of covalent character).
Aluminium fluoride on the other hand would remain ionic. This is because there is much less shielding effect as compared to the chloride ion. Hence, the electrostatic attraction between the nucleus and the valence electrons is greater, making it more difficult for the electron cloud to be distorted/”shared”.
Also, you should expect that other cations with high charge such as Fe3+ will form compounds with covalent character. Therefore, compounds such as FeCl3, FeBr3 and FeI3 are all covalent as well.
Some caveats:
Not much is known about aluminium astatide because astatine is a very unstable element. But if it is formed, you should expect that it has covalent character.
Theory predicts that aluminium sulphide (Al2S3) should be covalent in nature (See factor 2). But, it remains ionic. However, the reason and explanation for this is way beyond what can reasonably be tested on in the ‘O’ Levels. Hence, I will not discuss it here. If you are really interested or you just have too much free time, I suggest you visit Mr. David’s blog!
A (rather long) note about ozone layer and its depletion!
In this post I shall be explaining:
- Why absorbing too much ultraviolet radiation is harmful
- How the ozone layer protects us from absorbing too much ultraviolet radiation
- How the ozone layer is depleted by CFCs
Why absorbing too much ultraviolet radiation is harmful
When ultraviolet rays from the sun hits the skin, its energy is absorbed by the molecules in the skin causing the molecules in the skin to vibrate more vigorously. Sometimes, the UV ray is powerful enough such that it causes electrons to be detached from the molecules causing them to become ions.
This results in damage to the cells in the body. When the DNA in the cell is damaged, it may lead to abnormal pattern of cell division thus causing cancer.
However, UV rays do not carry the same amount of energy. Generally speaking, ultraviolet radiation from the sun can be divided into 3 bands, UVA, UVB, and UVC.
UVC has the highest energy as it has the highest frequency [thus UVC is the most harmful] while UVA has the lowest energy as it has the lowest frequency.
Fortunately, most of the UVB and UVC do not reach the Earth due to the ozone layer.
How does the ozone layer protect us from UVB and UVC?
Many students have the misconception that the ozone layer acts as some kind of giant shield that reflects the UV radiation back into outer space. This is not true. The ozone layer absorbs the UVC and UVB radiation as seen in the reaction sequence below.
Step 1:
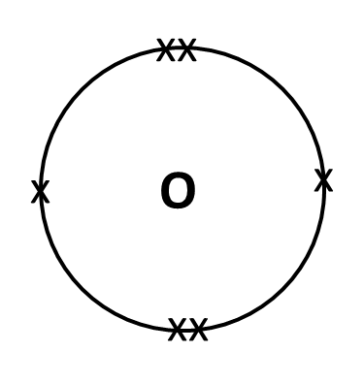
UVC which has the highest amount of energy is able to break the strong double covalent bond between the oxygen atoms in an oxygen molecule. (Remember, energy is absorbed during bond breaking!) This forms 2 oxygen atoms (The fancy JC term for this is called “free radical” – but this term just refers to any atom/molecule/ion with unpaired valence electrons.) The dot that you see beside the oxygen atom in the equation above is just to signify that the oxygen atom is a free radical, i.e. it has unpaired valence electrons. See below for electronic structure of oxygen atom.
Step 2:
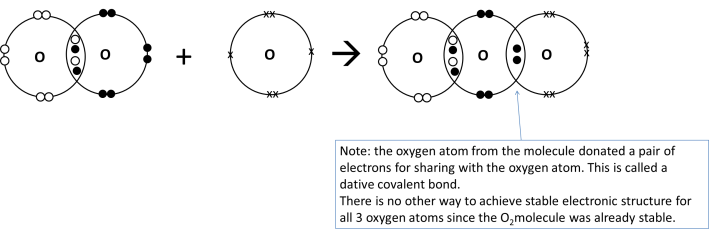
The oxygen atom is obviously unstable since it does not have a stable electronic structure, so it will react with another oxygen molecule to form ozone O3. The full equation with the various electronic structures is shown below
Step 3:
Ozone molecule is less stable as compared to oxygen molecule due to the dative covalent bond. Hence, the ozone molecule can be decomposed by the less energetic UVB radiation to form oxygen molecule and an oxygen atom (free radical).
Step 4:
The oxygen atom, in its quest for stability then reacts with another ozone molecule to form 2 oxygen molecules. And the cycle would repeat itself again from step 1.
There are 2 key points to note:
- Concentration of ozone in the atmosphere remains relatively stable. This is because, the 2 ozone molecules formed in step 2 are broken down in steps 3 and 4 respectively
- The continuous formation and destruction of ozone molecules helps absorb the harmful UVC and UVB radiation from the sun before it reaches the earth (Step 1 and 3).
How is the ozone layer depleted by CFCs?
Step 1:
At low altitudes, CFCs are stable, because the UVA rays that reach the earth’s surface do not have enough energy to break the C – Cl or the C – F bonds in CFCs. Therefore, the CFCs will diffuse to the higher altitudes. However, at high altitudes, the higher energy UVB/UVC rays are able to break the C – Cl bond to produce a chlorine atom. (Now, to be more precise there is a 2 stage reaction but I’m skipping one step to keep things short, since that step will not significantly alter the story anyway)
Step 2:
The chlorine atom is unstable and hence reacts with the ozone molecule to form oxygen gas and chlorine oxide which is free radical. See below for equation with electronic structures drawn in.
Step 3:
The chlorine oxide free radical reacts with another ozone molecule to form oxygen gas and a chlorine atom. Hence steps 2 and 3 will keep repeating resulting in a vicious cycle which destroys thousands of ozone molecules.
So, you may ask me, what is the problem? The problem is that steps 2 and 3 here result in the destruction of ozone molecules but without absorbing any harmful UVB unlike the previous mechanism! Hence, UVB radiation is are able to reach the earth and give sunbathers skin cancer.
This vicious cycle is only broken when the chlorine atom reacts with water vapour in the air to form hydrogen chloride which has a stronger bond and cannot be broken down by UVB or UVC radiation. (Note: There are other reactions that also remove the chlorine atom but let’s keep it simple)
Phew, we have finally reached the end of this long and convoluted story.. Thank you for taking the time and effort to read it! If you didn’t understand a thing, it’s alright; this story will not be tested in the exam! But it is an interesting story nonetheless, no? Hasta La Vista Baby!
Distinction between covalent bonds & Intermolecular bonds and how Mr affects m.p./b.p. of simple covalent substances
Covalent bond between atoms in a molecule: electrostatic force of attraction between the localized shared pair of electrons and the 2 positively charged nucleus. See diagram below
Intermolecular bonds: as the name suggests are bonds between the molecules. See diagram below
How do intermolecular bonds come about?
Even though we draw the dot-cross diagrams as shown above, please remember that electrons are not stationary but are always in constant random motion!
(Diagram above shows an electron cloud orbiting a nucleus.. Note that the continuously changing pattern shows random motion of the electrons! – For some reason, the gif is not moving.. will find a better pic soon..)
Therefore, at any point in time, there will always be an uneven distribution of electrons in a molecule. This results in one part of the molecule having a positive charge while the other part must have a negative charge. (The fancy A level term for this is instantaneous dipole)
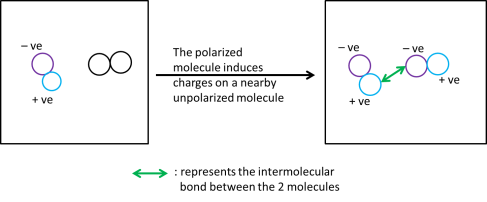
The instantaneous dipole in one molecule can cause the formation of dipoles in nearby unpolarized molecules.
As a result, a weak electrostatic attraction forms between the positive portion of one molecule and the negative portion of another molecule. This type of attraction is called the intermolecular bond/ Van Der Waals forces of attraction (fancy A level term = instantaneous dipole-induced dipole)
*Please note that these intermolecular bonds are fleeting in nature because the electrons are in constant motion. However, the process of bond forming and bond breaking keeps repeating such that on average, there will be attractive forces between the molecules.
How does Mr affect the strength of the intermolecular bonds?
1. The higher the Mr, the stronger the intermolecular bond (more important reason at O levels)
When Mr increases, number of protons and hence number of electrons increases. Hence, the dipoles formed by the random motion of electrons will, on average have larger +ve and larger –ve charges.
This increases the electrostatic forces of attraction between the positive portion of one molecule and the negative portion of the other molecule resulting in a stronger intermolecular bond.
2. Molecules with larger Mr have higher surface area and hence more dipole interactions (less important at O levels)
When Mr increases, the molecular size tends to increase due to increasing number of electron shells. Therefore, molecules have larger area in contact with other molecules. This increases the amount of the dipole interactions, hence forming more intermolecular bonds.
In turn, how does Mr affect m.p. and b.p. of a simple covalent substance?
Therefore, from the explanation above, higher Mr results in stronger intermolecular bonds which will require more energy to break. Therefore, m.p. and b.p. increases when Mr increases!
However, this is not the entire story. In the next post, I will discuss why some molecules with lower relative molecular masses have higher m.p./b.p. than molecules with higher relative molecular masses.
*Other Notes:
1. The above explanation only applies to simple covalent substances
2. Covalent bonds between the atoms are never broken during melting or boiling!!! (i.e. H2O after boiling still remains as H2O molecules just that the molecules are more widely spaced. It does not become hydrogen and oxygen atoms!) Therefore, simple covalent molecules have low m.p./b.p. because only the weak intermolecular bonds are broken during melting and boiling. The covalent bonds between the atoms which are very strong are not broken.